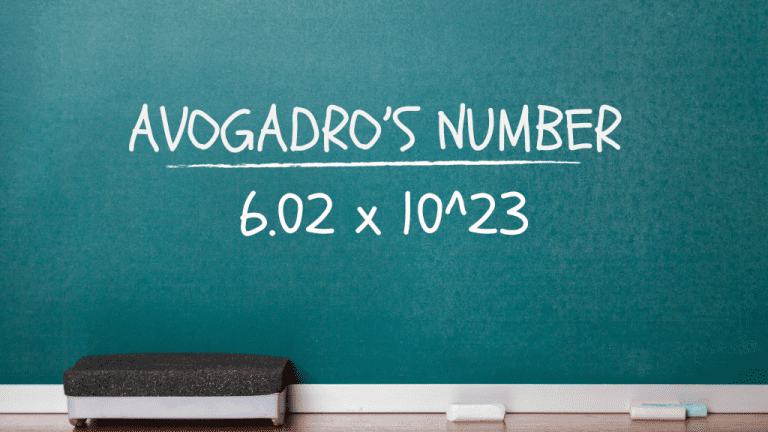X 10 23
May 14, 2025
Why was 6.02 x 10^23 taken as Avogadro's number? Why does the atomic mass of a gram of matter contain 6.02 x 10^23 molecules? - Quora 1.4 MOLES and AVOGADRO'S NUMBER | Easy, fast learn One mole of any substance contains `6.022xx10^(23)` atoms/molecules. Number of - YouTube Calculate the mass of 6.002 ×10^23 molecules of NH4cl | X 10 23