Novartis Product Recall
March 23, 2025
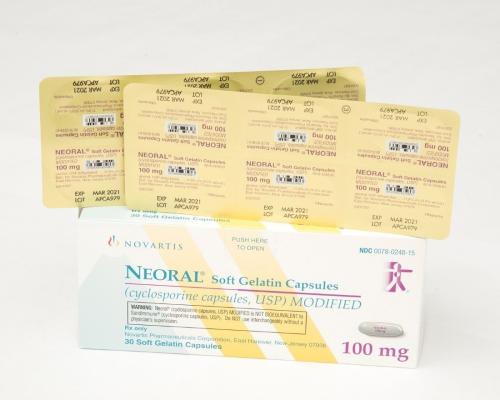
Closure failure behind Novartis OTC recall Broken products prompt voluntary Novartis recall | Packaging World Novartis Recalls 100 mg Sandimmune and Neoral Prescription Drug Blister Packages Due to Failure to Meet Child-Resistant Packaging Requirement; Risk of Poisoning | CPSC.gov Novartis, Sandoz recall 470,000 packs of prescription drugs | Miami Herald | Novartis Product Recall