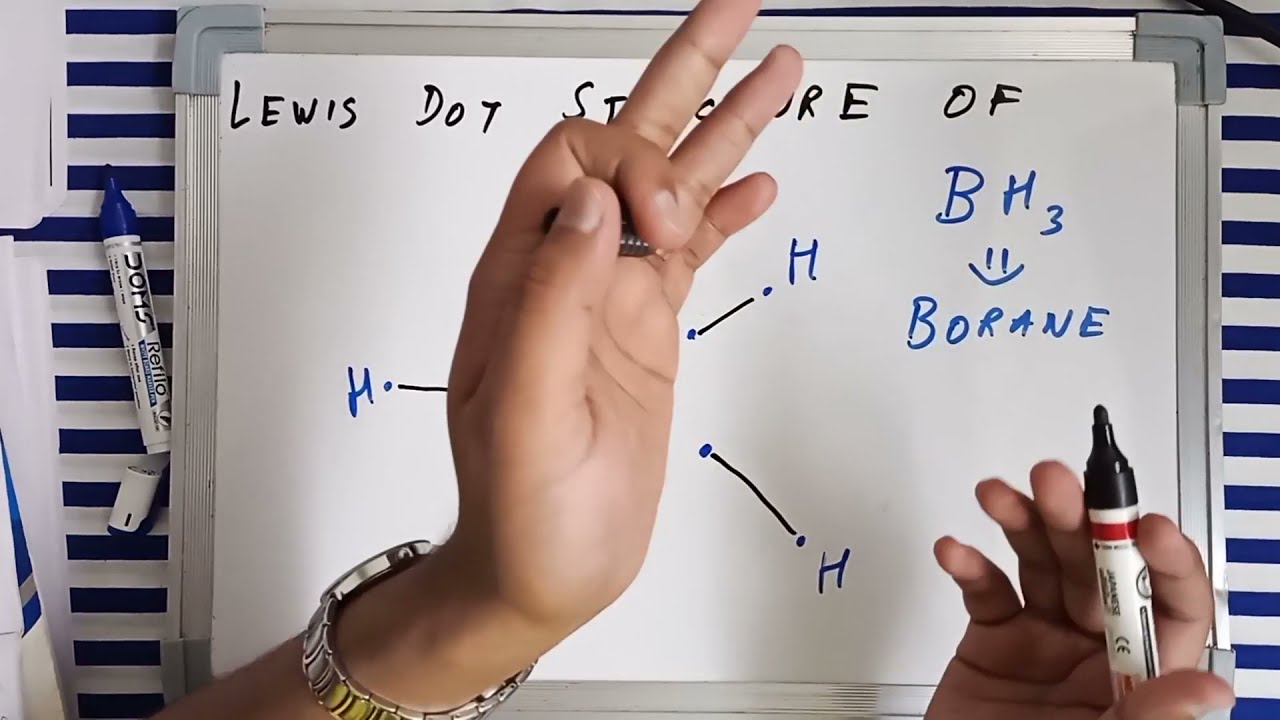
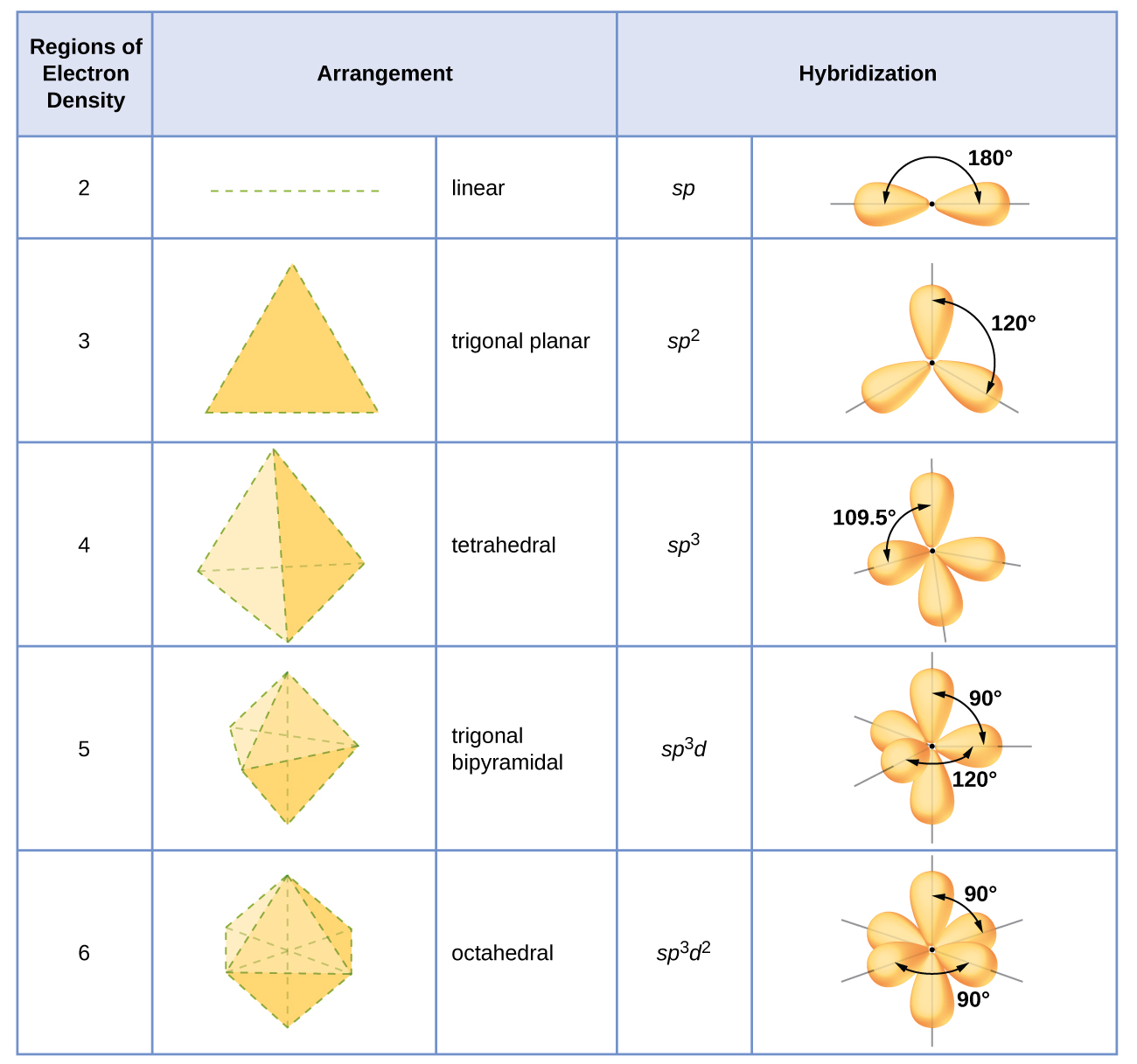
Bh3 Hybridization
March 29, 2025
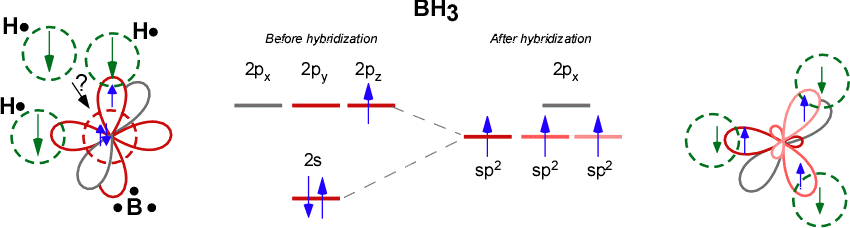
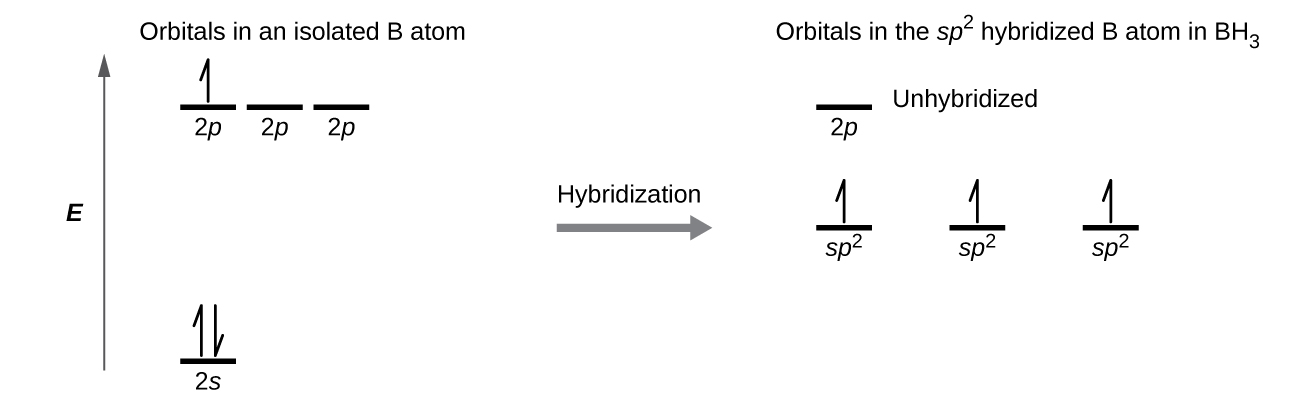
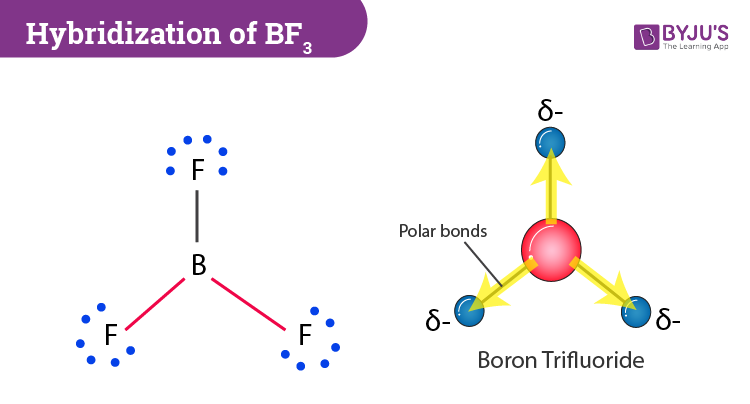
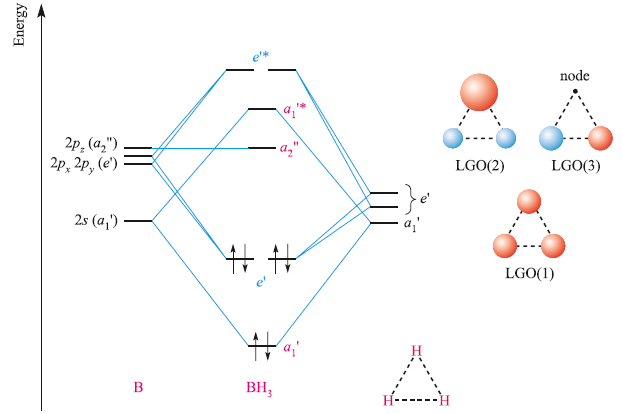
Discuss the orbital structures of the following molecules on the basis of hybridisation, (i) BH3 (ii) C2H2 - Science City - Quora The state of hybridization of the central atom in dimer of \\[B{H_3}\\] and \\[Be{H_2}\\] ?A. \\[s{p^2},s{p^2}\\]B. \\[s{p^3},s{p^2}\\]C. \\[s{p^3},s{p^3}\\]D. \\[s{p^2},sp\\] How to tell if a central element in a molecule needs to form hybridized orbitals? | Socratic The state of hybridisation of central atom in dimer of B{H}_{3} and Be{H}_{2}?s{ p }^{ 3 },s{ p }^{ 3 }s{ p }^{ 3 },s{ p }^{ 2 }s{ p }^{ 2 }, | Bh3 Hybridization
![Bh3 Hybridization 1262x792 The state of hybridization of the central atom in dimer of \\[B{H_3}\\] and \\[Be{H_2}\\] ?A. \\[s{p^2},s{p^2}\\]B. \\[s{p^3},s{p^2}\\]C. \\[s{p^3},s{p^3}\\]D. \\[s{p^2},sp\\]](https://www.vedantu.com/question-sets/258693db-7817-497f-a15b-137735afe7095512141555770833478.png)